Could someone explain the lewis structure diagram of covalent compound Al2Cl6?
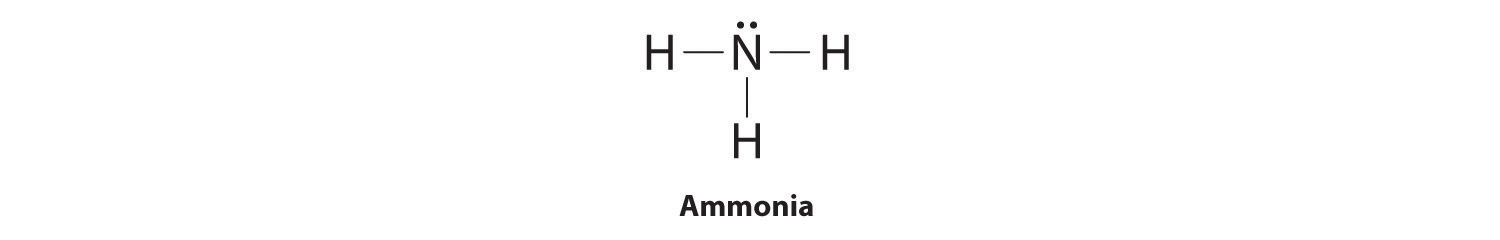
Here is the simple view on this important topic: In Lewis structures, we show each covalent bond as two dots which represent a pair of electrons. For example: Therefore, we can say that Lewis structures are electron dot representations for molecules. The trick here is to keep track of the electrons and correctly place the atoms in the molecule. Information: Steps for Drawing Lewis Structures for Covalent Compounds Study the two examples in the table of how to write structures for CO3 2-and NH3. Make sure you understand each of the five steps. CO3 2-NH 3 Step #1: Add up the number of valence electrons that should be included in the Lewis Structure. 4 + 3(6) + 2 = 24 (carbon has four.
It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for B–F single bonds. This suggests the best Lewis structure has three B–F single bonds. Drawing C shows just the atomic symbols and the shared pairs of electrons that constitute the four C-H covalent bonds. Drawing C is a Lewis electron dot structure for methane. Figure 1 Alternative Representations of Methane. Lewis Electron Dot Structures. Figure 2 animates the rules for drawing a Lewis electron dot structure using C 2 H 6 as an. Chemistry Worksheet Lewis Dot Structures Name: Block. Draw Lewis structures for the following covalent compounds: Created Date: 3/28/2014 11:29:01 AM.
Also, why is Al2Cl6 (aluminium chloride) covalent?
Here's what we have to know for school but I don't know how it works. What do the arrows mean?
Also, why is Al2Cl6 (aluminium chloride) covalent?
Here's what we have to know for school but I don't know how it works. What do the arrows mean?
1 Answer
Explanation:
Chlorine has 17 electrons, but 10 of those are in the orbitals of the lower energy levels. (#1S, 2S, 2P# Dxv codec for mac. orbitals).
These are completely enveloped by the larger #3S#-orbital (think of a golfball inside a tennisball) so takes no part in the formation of bonds.
The other seven are distributed in the #3S# and the three #3P#-orbitals, but upon forming (Covalent) bonds these orbitals hybridise into #SP#-orbitals
for more info about Hybridisation, here's a good link:
In our case the #3S#- and three #3P#-orbitals will hybridise into #SP^3#-orbitals.:
Covalent Lewis Dot Structure
(Picture courtesy of https://en.wikipedia.org/wiki/Orbital_hybridisation)
Each orbital can contain, and indeed strives to contain, 2 electrons (#e^-#).
Chlorine thus has 7 electrons in the 4 #SP^3#-orbitals: 3 orbitals are filled with 2 #e^-# each, the fourth has only one. If you look the the picture below (that I copied from Above) you will see 6 electrons paired in 3 'filled orbitals'
The fourth one, the one that contains the single, unpaired #e^-#, joins in the fourth #SP^3#-orbital with one from the Al-atom (the one on the right). So in this bond between the Al-atom and the Cl-atom, each donates a single electron. That's why the bond is represented by a straight line.
Aluminium has only 3 electrons: 2 in the #3S# and one in one of the #3P#-orbitals. However, upon hybridisation these 3 electrons are spread over 4 #SP^3#-orbitals. Like in the one mentioned above, the other two cooperate as well in the formation of covalent bonds with 2 Chlorine atoms These are circled in Green:
Chlorine has a rather high Electronegativity, which means that it pulls rather hard at electrons from other atoms (from each other, and from other elements.
It is a tug-of-war that the Aluminium atom is threatening to lose, leaving it rather #delta^+# (positive).
At the same time, the Chlorine atoms are satisfied in their hunger for electrons, in fact they don't want any more because they have a full set of 8!
Because of this 'diminished appetite' on the side of the Chlorine atoms, and the increased hunger on the part of the Aluminium atom, The bond is formed by BOTH of the Chlorine electrons.
This is the explanation for the arrow circled by Aqua:
Lewis Dot Structure Covalent Bonds Calculator Answer
In #Al_2Cl_6#, this happens twice but I'm sure you can spot the other one by now?
Hope this helps..
PS: By the way, it is covalent because the bonds are created by sharing of electrons between the two atoms.
Related questions
VIDEO explanation of the Covalent Bonds in an Electron Dot Structure (Lewis structure).
What is a covalent bond?
All following steps in creating and thinking about electron dot structures (Lewis structures) require that we first understand what a covalent bond is. Whenvalence electrons from different atoms come near each other they can form covalent bonds by sharing their electrons in an orbital between them. That means the electrons travel back and fourth between the two atoms. The picture below represents two atoms with their valence electrons just before they are about to join in a covalent bond.
How do we represent covalent bonds in Electron Dot Structures (Lewis structures)?
Keyboard shortcuts for pc and mac. Each single covalent bond is made up of 2 electrons.
We can represent a covalent bond in an electron dot structure (Lewis structure) by a blue line that connects the 2 valence electron red dots in our pictures. An example is below.
You can also create electron dot structures (Lewis structures) with more than one bond. This is known as creating double bonds or triple bonds. A double bond is just two bonds created between the same two atoms in an electron dot structure(Lewis structure). Example below.
A triple bond is just three bonds created between the same two atoms in an electron dot structure(Lewis structure). Example below.
PRACTICE PROBLEMS: Try drawing for yourself single, double, and triple bonds in structures. Start with the picture I have provided and then complete it.
Cl2 has a single bond between the two atoms.
Se2 has a double bond between the two atoms.
As2 has a triple bond between the two atoms.
